GRA Eminent Scholar named to lead Georgia’s Solve Sickle Cell Initiative
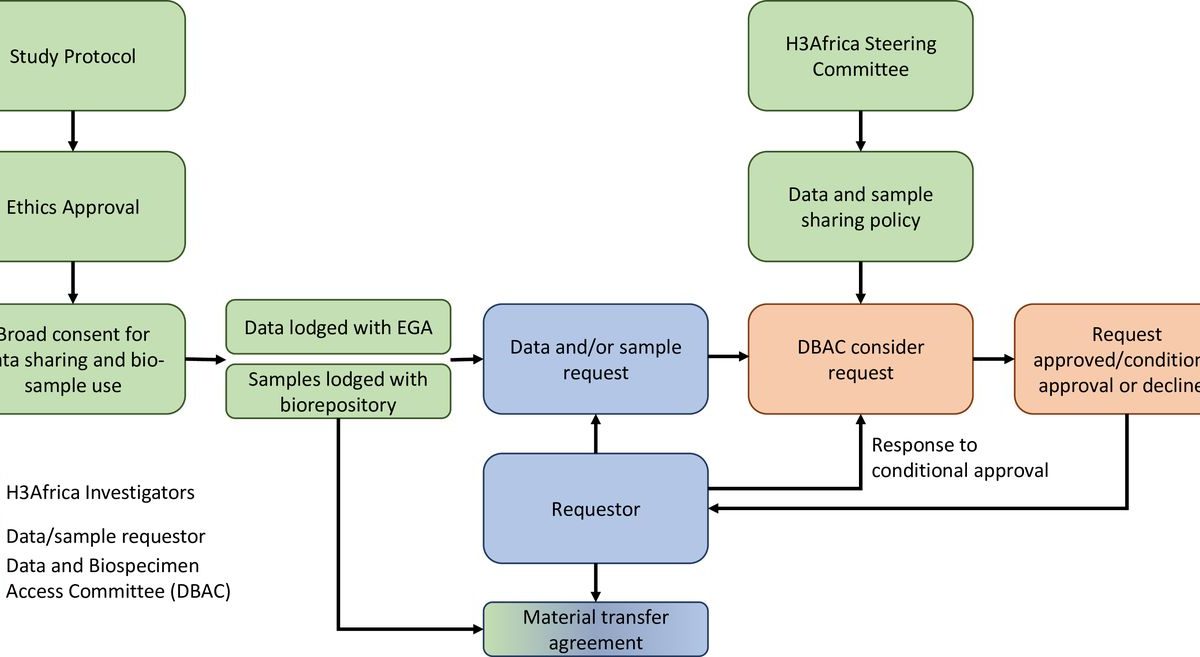
August 14, 2024International collaboration in genomic research is gaining momentum in African countries and is often supported by external funding. Over the last decade, there has been an increased interest in African genomic data. The contribution of this rich data resource in understanding diseases predominant in both African and global populations has been limited to date. There has been some non-governmental funding dedicated to the advancement of genomic research and innovation by African-based and African-led research groups, but the impact of these initiatives is hard to quantify. However, there is now an opportunity for the global research community to leverage decades of genomic data and biospecimens originating from African populations. The experience we describe in this paper is of an access governance framework established under the Human, Heredity, and Health in Africa (H3A) consortium, given the task of managing wider access to the data and biospecimen resources collected via its various projects. The function of the Data and Biospecimen Access Committee (DBAC) is to facilitate the advancement of medicine and health while fostering the development of bioinformatics capabilities at Africa-based institutions or regional hubs. Our collective experiences and lessons learnt as a committee provide examples of nuanced considerations when evaluating access to African data. The committee was semi-autonomous in its establishment and had independence in decision-making. The DBAC continually advocates for the responsible use of genomic data and biospecimens that were obtained from African research participants, under broad consent, by primary researchers who no longer have oversight over the future use of these resources.